News
-

How does the passivation effect impact self-discharge rates in batteries?
How does the passivation effect impact self-discharge rates in batteries?View -
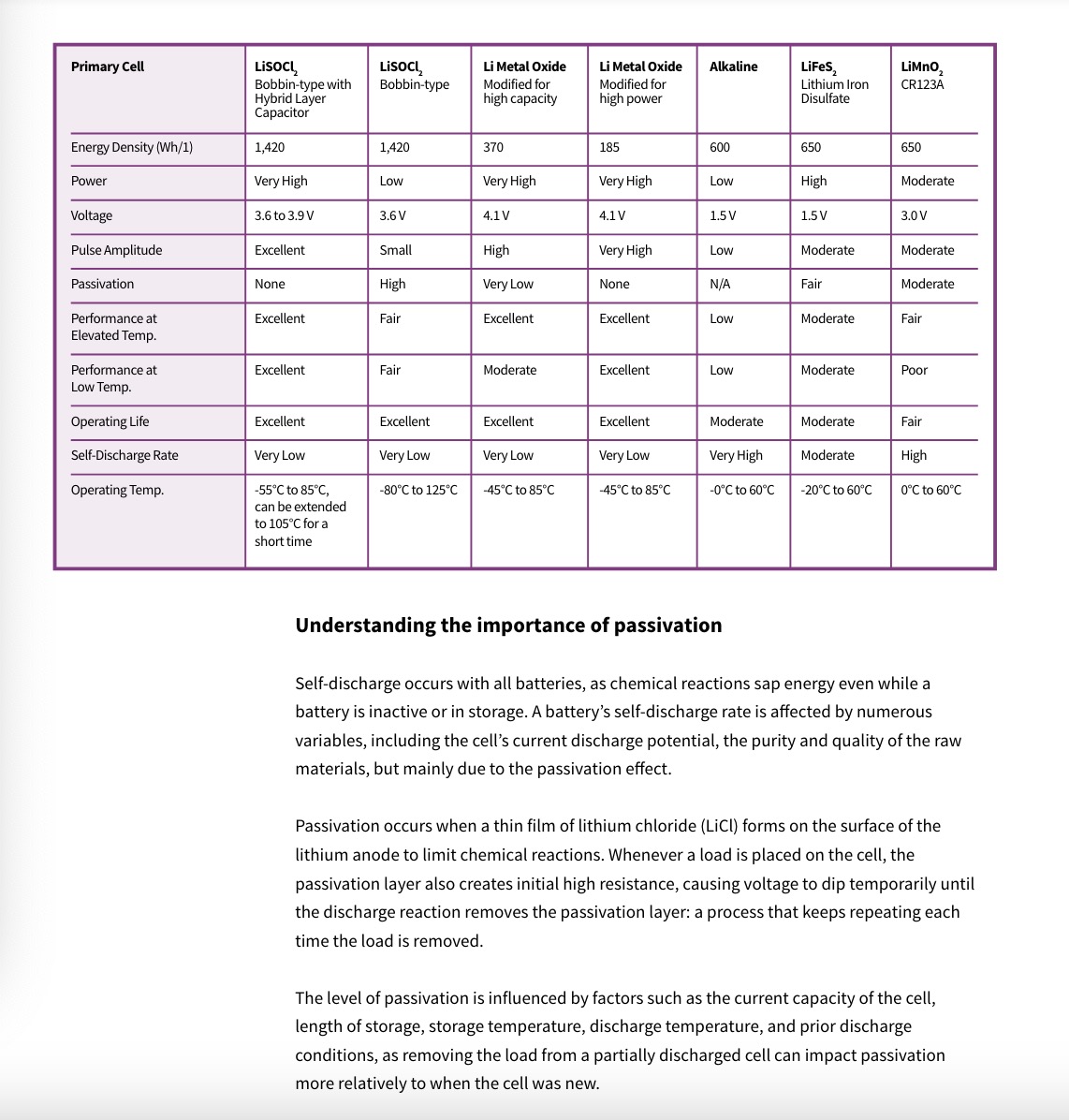
Understanding the passivation effect
A little known chemical reaction is essential to extended battery life As remote wireless devices become ubiquitous to the Industrial Internet of Things (IIoT), there is a growing need to extend battery life, especially for applications involving long-term deployments in hard-to-access locations and extreme environments. Certain low-power devices draw average current measurable in microamps with high pulses measurable in the multi-amp range. These devices are carefully designed to conserve as much energy as possible to operate for decades on their original battery. This requirement limits the choice of primary batteries that can be used to deliver long-term power. Within the lithium family there are a number of primary (non-rechargeable) chemistries, including iron disulfate (LiFeS2 ), lithium manganese dioxide (LiMnO2 ), lithium thionyl chloride (LiSOCl2 ), and lithium metal-oxide. (See table on next page) Of all these choices, lithium thionyl chloride (LiSOCl2 ) batteries are overwhelmingly chosen for long-term deployments because they deliver the highest capacity and highest energy density of all lithium cells to support product miniaturization. Bobbin-type LiSOCl2 cells also feature an incredibly low self-discharge rate as low as 0.7% per year, largely due to harnessing the passivation effect, enabling certain low-power devices to work up to 40 years on the original battery.View -

Use the Right Battery for Remote devices sensors IoT devices
Highdrive LI-SOCL2 battery Lithium thionyl chloride Battery-powered devices provide an economical means for expanding the Industrial Internet of Things (IIoT) beyond the electrical power grid to virtually anywhere at reasonably low costView -

How to Removal of passivation method for lithium thionyl chloride battery
Removal of passivation method for lithium thionyl chloride battery ER34615View -

The ways of Lisocl2 battery +SPC as a system for IoT devices
Battery Power Supply Solution for IoT GSM GPRSView -

LI-SOCL2 battery hysteresis generation and recommended solutions
LI-SOCL2 battery hysteresis generationView -
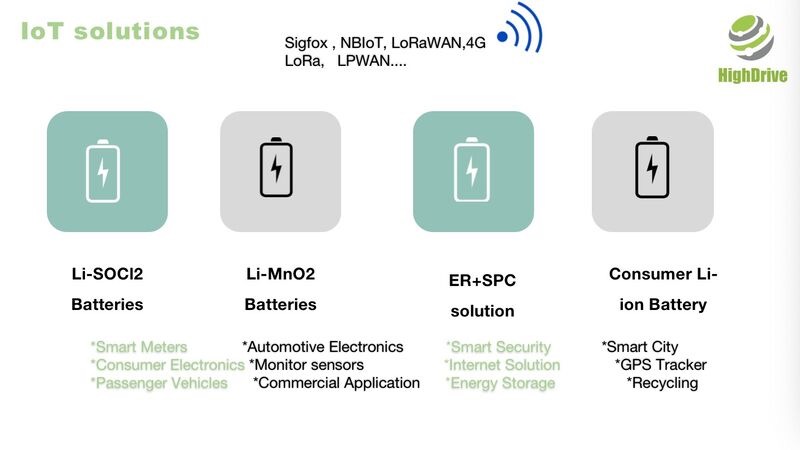
IoT battery,low power battery
Choosing the right battery for your smart deviceView -

Shenzhen Highdrive Tech Co., Ltd
★ Shenzhen Highdrive Tech Co., Ltd is battery factory Since 2014 ,which can customized as customer's requirements.View -
LI-SOCL2 battery hysteresis generation and recommended solutions
When Li contacts SOCl2, the following chemical reactions will occurView -

The ways of Lisocl2 battery +SPC as a system for IoT devices
Power Supply SolutionView -

How to Removal of passivation method for lithium thionyl chloride battery ER34615
How to Removal of passivation method for lithium thionyl chloride batteryView -

How can you identify a high-quality Battery manufacturer
How can you identify a high-quality Battery manufacturerView



